Abstract
Introduction: ASXL2 (additional sex combs like 2) gene is required for normal hematopoiesis with non-overlapping, distinct effects from ASXL1 and acts as a haploinsufficient tumor suppressor (Micol et. al, 2017, Nature Comm.). While there has been interest in ASXL1 due to its frequent mutation in myeloid neoplasms (MN), little is known about its paralog ASXL2. Here, we identify the frequency, clinical characteristics, and mutational cooccurrences of ASXL2 in patients (pts) with MNs, including AML, myelodysplastic syndrome (MDS), myeloproliferative neoplasms (MPN).
Methods: We identified 5746 pts who were presented with MNs, including AML (N=2473), MDS (N=1477), MPN (N=1299), MDS/MPN overlap neoplasms (N=497), to our institution between January 2017 through July 2022. All pts had next generation sequencing (NGS) performed by a clinical-grade myeloid gene panel (81-gene panel) using the Illumina MiSeq upon presentation to our institution. A minimum of 250× coverage with a detection sensitivity of ∼5% was used for variant calling.
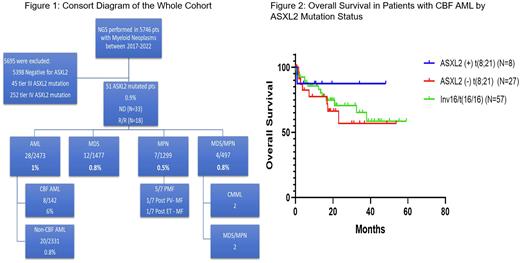
Results: Fifty-one patients (0.9%) were identified to have ASXL2 mutations (Figure1, Consort Diagram). The median age was 65 years (range, 18.8-94.2) with a slight male predominance (59%). Thirty-three pts were newly diagnosed (ND) (65%) and the rest (35%) had relapsed refractory disease (R/R).
Of the 51 pts, 8 (6%), 20 (0.8%), 12 (0.8%), 7 (0.5%), and 4 (1%) had CBF- AML, non-CBF AML, MDS, MPN, and MDS/MPN, respectively. In the entire cohort, RAS (22%), SRSF2 (20%), TET2 (14%), and DNMT3A (14%), SF3B1 (12%), RUNX1 (12%), were the most commonly co-mutated genes. Six pts had co-existing ASXL1 mutations; 3in AML, 2 in MPN and 1 MDS.
Acute Myeloid Leukemia
Of the 28 pts with ASXL2 mutated AML, 16 and 12 were newly diagnosed and relapsed/refractory. Eight of 16 ND pts had CBF AML, and the remining 8 ND pts had non-CBF AML. We identified somatic mutations in ASXL2 in 15% (8/53) of pts with t(8;21), but not in pts with inv(16)/t(16;16) (0/57) (Figure 2). In frontline setting, the response rates and median overall survival rates (OS) were 100% and 87%, not reached and 12.4 months in CBF (all pts received FLAG based chemotherapy) and non-CBF AML (2 pts received intensive Rx, 6 pts received low-intensity Rx) respectively.
All 12 R/R patients had non CBF AML, the response rates after salvage chemotherapy and median overall survival rates (OS) were 67% and 6.2 months (22% intensive Rx, 78% low-intensity Rx), respectively. The most commonly co-mutated genes in AML were: SRSF2 (18%), IDH2 (18%), RUNX1 (14%), SF3B1 (14%), KIT (14%) and DNMT3A (14%).
Myelodysplastic Syndrome
Of the 12 pts with ASXL2 mutated MDS, 9 and 3 were ND and R/R disease. In ND pts, most (58%) had intermediate risk IPSS (3 low, 7 intermediate, 2 high). Of the 9 pts, 6 received HMA based therapy (4 had response -2 CR and 2 mCR- and 2 no response,), 1 lenalidomide (achieved response). The median OS was poor, only 12.9 months in these pts with ND MDS. Most common associated mutations were SRSF2 in 4 pts (33%),TP53 in 3 pts (25%) 1 pt had cooccurring ASXL1 mutations.
Myeloproliferative Neoplasm
All 7 pts had myelofibrosis (MF) (5 primary MF, 1 post essential thrombocytosis MF and 1 post Polycythemia-Vera MF). Five (71%) pts harbored JAK2 V617F mutation, 2 (29%) MPL mutation and 3 (43%) ASXL1 mutation. Six out of 7 patients had intermediate risk based on DIPSS score (4 Intermediate-1 and 2 intermediate-2 risk). The 3-year OS rate was 100%.
MDS/MPN Overlap Neoplasms
Of the 4 pts with MDS/MPN, 2 pts had ND chronic myelomonocytic leukemia (CMML, 2 achieved CR and 1 relapsed),1 ND MDS/MPN unclassified (achieved CR with decitabine and then progressed to AML) and 1 R/R MDS/MPN with fibrosis (died from COVID-19 infection prior to salvage therapy).
Conclusion: With the exception of CBF AML, ASXL2 mutations are infrequent in myeloid malignancies. In CBF AML, ASXL2 mutations were restricted to pts bearing the t(8;21) / RUNX1-RUNX1T1 (AML1-ETO) fusion. ASXL2 mutations seemed to have no negative prognostic impact in CBF AML and MF. Due to the rarity of ASXL2 mutations in MDS and MDS/MPN overlap neoplasms, its prognostic impact in these disease groups remains unclear.
Disclosures
Verstovsek:Protagonist Therapeutics: Research Funding; Promedior: Research Funding; Novartis: Consultancy, Research Funding; Genentech: Research Funding; CTI BioPharma Corp.: Research Funding; ItalPharma: Research Funding; Gilead: Research Funding; Incyte: Consultancy, Research Funding; PharmaEssentia: Research Funding; NS Pharma: Research Funding; Sierra Oncology: Consultancy, Research Funding; Roche: Research Funding; Constellation Pharmaceuticals: Consultancy; Celgene: Consultancy, Research Funding; Blueprints Medicines Corp.: Research Funding; AstraZeneca: Research Funding; Pragmatist: Consultancy. Borthakur:Catamaran Bio, Abbvie, PPD Development, Protagonist Therapeutics, Janssen: Consultancy; Pacylex, Novartis, Cytomx, Bio Ascend: Membership on an entity's Board of Directors or advisory committees; Astex Pharmaceuticals, Ryvu, PTC Therapeutics: Research Funding. Kanagal-Shamanna:Amgen: Consultancy; Novartis: Consultancy; Aptitude Health: Speakers Bureau; Physicians Education Resource: Speakers Bureau. Takahashi:Symbio Pharmaceuticals: Consultancy; Novartis: Consultancy; Celgene/BMS: Consultancy; GSK: Consultancy; Agios: Consultancy; Ostuka Pharmaceuticals: Honoraria; Mission Bio: Honoraria; Illumina: Honoraria. Bose:AbbVie: Consultancy; Sierra Oncology (now GSK): Consultancy; Promedior: Research Funding; Novartis: Honoraria; Pfizer: Research Funding; Astellas: Research Funding; Telios: Research Funding; Disc Medicine: Research Funding; NS Pharma: Research Funding; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Honoraria, Research Funding; BMS: Consultancy, Research Funding; Karyopharm: Consultancy; Incyte: Honoraria, Research Funding; Pharma Essentia: Honoraria; Ionis: Research Funding; Kartos: Research Funding; Cogent: Honoraria, Research Funding; Blueprint Medicines Corporation: Honoraria, Research Funding; CTI BioPharma: Honoraria, Research Funding. Garcia-Manero:Curis: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Aprea: Honoraria; AbbVie: Honoraria, Research Funding; Acceleron Pharma: Consultancy; Novartis: Honoraria, Research Funding; Gilead Sciences: Research Funding; BMS: Consultancy, Honoraria, Research Funding; Astex: Consultancy, Honoraria, Research Funding. Short:Takeda Oncology: Consultancy, Research Funding; Astellas: Research Funding; Pfizer: Consultancy; Amgen: Consultancy, Honoraria; AstraZeneca: Consultancy; Novartis: Consultancy; Stemline Therapeutics: Research Funding. DiNardo:ImmuneOnc: Honoraria, Research Funding; Astellas: Honoraria; Jazz: Honoraria; Gilead: Honoraria; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Kura: Honoraria, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; GenMab: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Research Funding; Bluebird Bio: Honoraria; Cleave: Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Foghorn: Honoraria, Research Funding; Takeda: Honoraria; Forma: Research Funding; LOXO: Research Funding; Astex: Research Funding; Novartis: Honoraria; Servier: Consultancy, Honoraria, Research Funding. Daver:Agios, Celgene, SOBI and STAR Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kartos and Jazz Pharmaceuticals: Other: Data monitoring committee member; Karyopham Therapeutics and Newave Pharmaceutical: Research Funding; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Novartis, Jazz, Amgen, Servier, Karyopharm, Trovagene, Trillium, Syndax, Gilead, Pfizer, Bristol Myers Squibb, Kite, Actinium, Arog, Immunogen, Arcellx, and Shattuck: Consultancy, Other: Advisory Role; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Gilead, Immunogen, Pfizer, Bristol Myers Squibb, Trovagene, Servier, Novimmune, Incyte, Hanmi, Fate, Amgen, Kite, Novartis, Astex, KAHR, Shattuck, Sobi, Glycomimetics, Trillium: Research Funding. Ravandi:Astex/Taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; Biomea Fusion, Inc.: Research Funding; Astellas: Consultancy, Honoraria, Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Prelude: Research Funding; Novartis: Consultancy; Abbvie: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Xencor: Research Funding; Syos: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy. Kantarjian:Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Research Funding; KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Research Funding; NOVA Research: Honoraria; Daiichi-Sankyo: Consultancy, Research Funding; ImmunoGen: Research Funding; Novartis: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Research Funding; Takeda: Honoraria. Yilmaz:Daiichi-Sankyo: Research Funding; Pfizer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal